Isomerism
Isomers are molecules with the same molecular formula, but different structural formulae.
There are 3 types of isomerism you will be concerned with at A-Level.
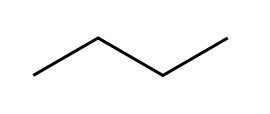
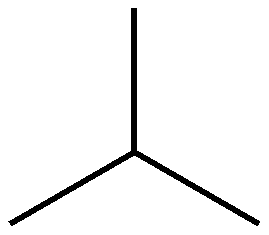
1. Structual Isomerism-The constituent atoms are bonded in different places, For example butane C4H10 and methlpropane C4H10 where the only difference is the placement of the 4th carbon. 2. Cis/Trans Isomerism. This occurs around double bonds (usually C=C) which cannot freely rotate, meaning that the positions of the groups coming off of these carbons are fixed relative to each other. As such the positioning of these groups can radically affect the shape and thus the properties of molecules These molecules are prefixed with cis if the two largest groups form a U shape (as on the left) and trans-if they form a Z-shape as on the right. This is also sometimes called E/Z Isomerism with E (From the German entgegen meaning opposite) being the same as trans (the Z shape) and Z (from zusammen, meaning together) being the same as cis (the C shape). This is counter-intuitive, and a perfect example of why the Germans shouldn't be allowed near language. 3. Optical Isomerism This occurs when a carbon has 4 different groups bonded to it. When this happens, it is possible to change the order of these groups to make 2 completely different chemicals. These two chemicals will be mirror images of each other, but no matter how they are rotated they can never be superimposed (a good analogy is human hands, as the left and right hands are mirror images, but no matter how you turn them they cannot be made to overlap). These are called optical isomers because when plane polarised light is shone through a sample of one isomer, it is rotated in the opposite direction to the rotation caused by the other isomer. This is also known as chirality and the central carbon is often referred to as the chiral carbon. |
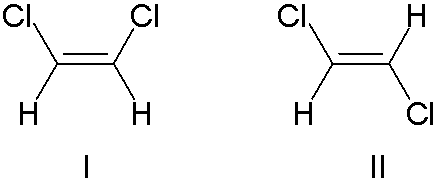 I Cis-1,2-Dichloroethene II Trans-1,2-dichloroethene 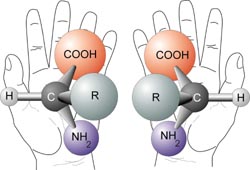 Two optical isomers (Generic amino-acids) |
Thanks to Wikipedia, NASA, and V8rik for images. All images are either public domain or subject to GNU licencing.